计算物理 ›› 2023, Vol. 40 ›› Issue (4): 453-460.DOI: 10.19596/j.cnki.1001-246x.8591
刘启帆1( ), 闫好奎2, 布玛丽亚·阿布力米提1,*(
), 闫好奎2, 布玛丽亚·阿布力米提1,*( ), 向梅1,*(
), 向梅1,*( ), 安桓1, 郑敬严1
), 安桓1, 郑敬严1
收稿日期:2022-07-13
出版日期:2023-07-25
发布日期:2023-10-13
通讯作者:
布玛丽亚·阿布力米提, 向梅
作者简介:刘启帆(1998-),男,硕士研究生,主要从事分子反应动力学方面的研究, E-mail:308698499@qq.com
基金资助:
Qifan LIU1( ), Haokui YAN2, Abulimiti BUMALIYA1,*(
), Haokui YAN2, Abulimiti BUMALIYA1,*( ), Mei XIANG1,*(
), Mei XIANG1,*( ), Huan AN1, Jingyan ZHENG1
), Huan AN1, Jingyan ZHENG1
Received:2022-07-13
Online:2023-07-25
Published:2023-10-13
Contact:
Abulimiti BUMALIYA, Mei XIANG
摘要:
利用密度泛函理论在B3LYP/6-311+G(d, p)水平上研究不同外加电场下(-0.05~0.05 a.u.)一氟二氯乙烷分子的光谱特征和解离特性, 其中包括该分子基态结构、总能量、偶极矩、最高占据轨道能级、最低空轨道能级、能隙、红外光谱、拉曼光谱、紫外可见光吸收光谱及C原子与Cl原子间势能曲线等数据在电场下的变化趋势。在y轴方向上, 随着负向电场的增强, C原子与Cl原子核间距增长、最高占据轨道能级减小, 体系总能量、最低未占据轨道能级、能隙先增大后减小, 偶极矩先减小后增加。外加电场会影响一氟二氯乙烷分子红外光谱、拉曼光谱与紫外可见光吸收光谱的吸收强度和吸收峰频率, 红外光谱、拉曼光谱与紫外可见光吸收光谱随着电场变化出现红移或蓝移现象。C原子与Cl原子间势垒随着负向电场增加逐渐减小, 并在电场达到-0.05 a.u.时C原子与其中一个Cl原子发生断裂, 当分子中一个C—Cl键断裂后, 施加强度为-0.04 a.u.的电场时另外一个C—Cl键发生断裂, 分子在电场下发生逐步解离, 研究结果完善了一氟二氯乙烷分子受外电场影响的理论数据。
刘启帆, 闫好奎, 布玛丽亚·阿布力米提, 向梅, 安桓, 郑敬严. 一氟二氯乙烷分子在外电场中的光谱特征和解离特性[J]. 计算物理, 2023, 40(4): 453-460.
Qifan LIU, Haokui YAN, Abulimiti BUMALIYA, Mei XIANG, Huan AN, Jingyan ZHENG. Spectral Characteristics and Dissociation Characteristics of Monofluorodichloroethane Molecule in External Electric Field[J]. Chinese Journal of Computational Physics, 2023, 40(4): 453-460.
| Method | RC—F/nm | RC—Cl/nm |
| B3LYP/6-311+G (d, p) | 0.136 | 0.180 |
| MPW1PW91/6-311+G (d, p) | 0.135 | 0.178 |
| MPW1PW91/3-21G | 0.136 | 0.187 |
| BYV86/6-311++G (d, p) | 0.137 | 0.181 |
| Ref.[ | 0.134 | 0.177 |
表1 不同基组计算得到的HCFC-141b分子稳定构型参数
Table 1 Stable configuration parameters of HCFC-141B molecule calculated by different basis groups
| Method | RC—F/nm | RC—Cl/nm |
| B3LYP/6-311+G (d, p) | 0.136 | 0.180 |
| MPW1PW91/6-311+G (d, p) | 0.135 | 0.178 |
| MPW1PW91/3-21G | 0.136 | 0.187 |
| BYV86/6-311++G (d, p) | 0.137 | 0.181 |
| Ref.[ | 0.134 | 0.177 |
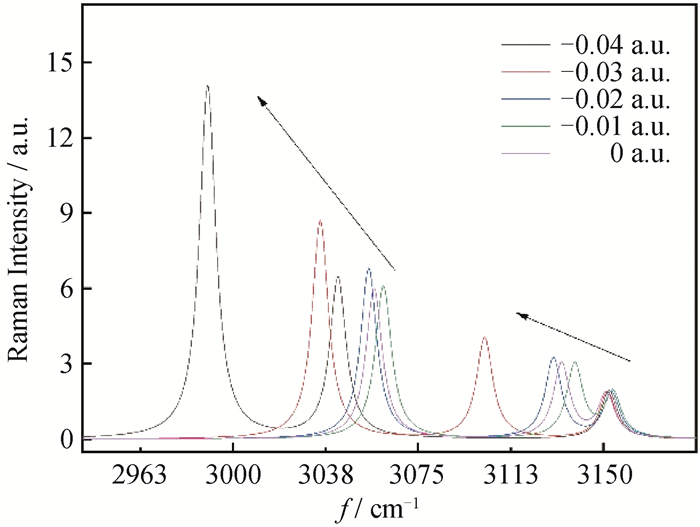
图9 Raman光谱在3057.06、3132.98、3151.14 cm-1处吸收峰随负向电场的变化
Fig.9 Raman spectrum 3057.06、3132.98、3151.14 cm-1 absorption peak changes with negative external electric field
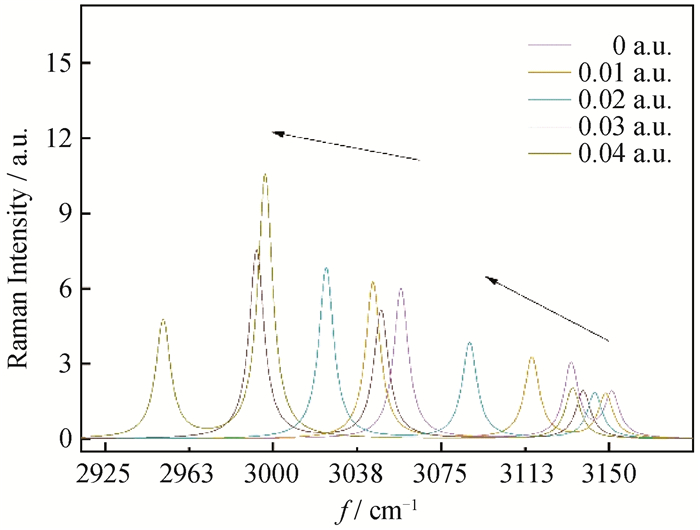
图10 Raman光谱在3057.06、3130.98、3151.14 cm-1处吸收峰随正向电场的变化
Fig.10 Raman spectrum 3057.06、3130.98、3151.14 cm-1 absorption peak changes with forward external electric field
| 1 |
|
| 2 |
|
| 3 |
|
| 4 |
DOI |
| 5 |
DOI |
| 6 |
DOI |
| 7 |
DOI |
| 8 |
DOI |
| 9 |
DOI |
| 10 |
DOI |
| 11 |
DOI |
| 12 |
|
| 13 |
李曾婷. 2019年1月1日起冰箱、电热水器行业全面禁止使用HCFC-141b[J]. 电器, 2018, 11, 49.
|
| 14 |
DOI |
| 15 |
|
| 16 |
|
| 17 |
|
| 18 |
DOI |
| 19 |
DOI |
| 20 |
|
| 21 |
DOI |
| 22 |
DOI |
| 23 |
DOI |
| 24 |
|
| 25 |
|
| 26 |
|
| 27 |
|
| 28 |
|
| 29 |
|
| 30 |
FRISCH M J, TRUCKS G W, SCHLEGEL H B, et al. Gaussian 09, Revision D. 01[CP]. Wallingford CT: Gaussian Inc, 2009.
|
| 31 |
|
| [1] | 詹泓飞, 蔡振宁, 胡光辉. 基于虚时间演化与谱方法的一类基态Wigner函数计算方法[J]. 计算物理, 2022, 39(6): 651-665. |
| [2] | 乃皮赛·吾买尔江, 闫好奎, 布玛丽亚·阿布力米提, 王丹琦, 向梅, 安桓. 外电场下CHBr3分子的光谱和解离特性[J]. 计算物理, 2022, 39(5): 624-630. |
| [3] | 吉航, 孙仲谋, 周卓彦, 刘玉柱. 外电场对CO分子及离子性质的调制和降解[J]. 计算物理, 2022, 39(3): 327-334. |
| [4] | 高慧, 杨在发, 赵敬芬, 袁慧敏, 刘志娥, 赵显. 第一性原理研究Nb、Sn、Cu、Fe和Cr对Zr (0001)晶面抗疖状腐蚀性能的影响[J]. 计算物理, 2022, 39(1): 101-108. |
| [5] | 周卓彦, 刘玉柱, 陈宇, 张昕阳, 孙卓怡. 丙烯醛在外电场中的解离性质[J]. 计算物理, 2021, 38(6): 722-728. |
| [6] | 赵一程, 郭俊宏, 胡芳仁. 应变对单层砷烯结构拉曼散射的影响[J]. 计算物理, 2020, 37(3): 365-370. |
| [7] | 柴汝宽, 刘月田, 杨莉, 张艺馨, 辛晶, 马晶. 两种不同极性有机小分子在方解石(104)面吸附的密度泛函研究[J]. 计算物理, 2020, 37(2): 221-230. |
| [8] | 陈余, 邢永明. 静水压力对Al14Mn2P16磁光性质影响的密度泛函理论研究[J]. 计算物理, 2020, 37(2): 231-239. |
| [9] | 尹海峰, 曾春花, 陈文经. 二维二元碳化硅纳米结构的等离激元激发[J]. 计算物理, 2019, 36(5): 603-609. |
| [10] | 何志伟, 张秀荣. (BN)25团簇的结构与性能[J]. 计算物理, 2019, 36(2): 219-224. |
| [11] | 韩晓琴, 肖夏杰. POX(X=1,2)的光谱常数与从头算势能曲线[J]. 计算物理, 2019, 36(1): 106-112. |
| [12] | 周康, 冯庆, 田芸, 李科, 周清斌. 过渡金属Cu、Cr掺杂TiO2表面氧化性气体NO2光学气敏传感特性[J]. 计算物理, 2018, 35(6): 702-710. |
| [13] | 王啸卿, 刘玉柱, 尹文怡, 李金花. 电场作用下溴甲烷的光谱和解离特性[J]. 计算物理, 2018, 35(5): 619-625. |
| [14] | 伍冬兰, 谭彬, 温玉锋, 曾学锋, 谢安东. 基于多参考组态方法研究MgI分子激发态的光谱[J]. 计算物理, 2018, 35(4): 469-474. |
| [15] | 谢建明, 陈红霞, 庄国策. Mn掺杂(ZnSe)12团簇物性研究[J]. 计算物理, 2018, 35(4): 481-486. |
| 阅读次数 | ||||||
|
全文 |
|
|||||
|
摘要 |
|
|||||
版权所有 © 《计算物理》编辑部
地址:北京市海淀区丰豪东路2号 邮编:100094 E-mail:jswl@iapcm.ac.cn
本系统由北京玛格泰克科技发展有限公司设计开发
