Chinese Journal of Computational Physics ›› 2023, Vol. 40 ›› Issue (6): 699-711.DOI: 10.19596/j.cnki.1001-246x.8678
Previous Articles Next Articles
Qingzhou ZHANG1,2( ), Dawei FAN1,2, Linghong LIU1,2,*(
), Dawei FAN1,2, Linghong LIU1,2,*( )
)
Received:2022-12-12
Online:2023-11-25
Published:2024-01-22
Contact:
Linghong LIU
CLC Number:
Qingzhou ZHANG, Dawei FAN, Linghong LIU. First-principles Study on the Influence of Alloying Elements on Galvanic Corrosion of Ternary L12-Al-Zr-X Aluminum Alloys Surface[J]. Chinese Journal of Computational Physics, 2023, 40(6): 699-711.
Add to citation manager EndNote|Ris|BibTeX
URL: http://www.cjcp.org.cn/EN/10.19596/j.cnki.1001-246x.8678
| Surface | W/eV | Wexp/eV | Esurf/(J·m-2) | ||
| (100) | 4.34 | 4.30[ | 4.41[ | 1.03 | 0.96[ |
| (110) | 4.09 | 4.09[ | 4.28[ | 1.04 | 1.02[ |
| (111) | 4.02 | 4.02[ | 4.24[ | 0.84 | 0.73[ |
Table 1 Work function (W) and surface energy (Esurf) of different surfaces of Al matrix
| Surface | W/eV | Wexp/eV | Esurf/(J·m-2) | ||
| (100) | 4.34 | 4.30[ | 4.41[ | 1.03 | 0.96[ |
| (110) | 4.09 | 4.09[ | 4.28[ | 1.04 | 1.02[ |
| (111) | 4.02 | 4.02[ | 4.24[ | 0.84 | 0.73[ |
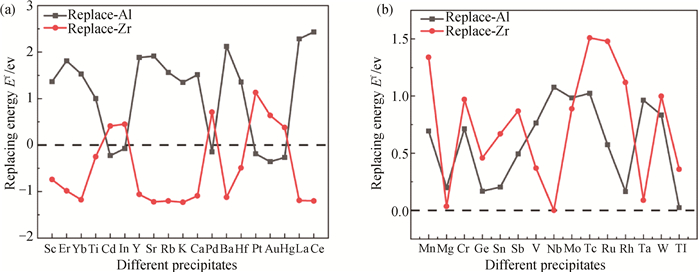
Fig.1 Substitution formation enthalpy Ef of the Al/Zr sites in the precipitated phase of L12-Al3Zr binary by X atoms (the dotted line indicates Ef=0) (a) the substitution of Al/Zr atoms by X atom; (b) the substitution of Al/Zr atoms not by X atoms
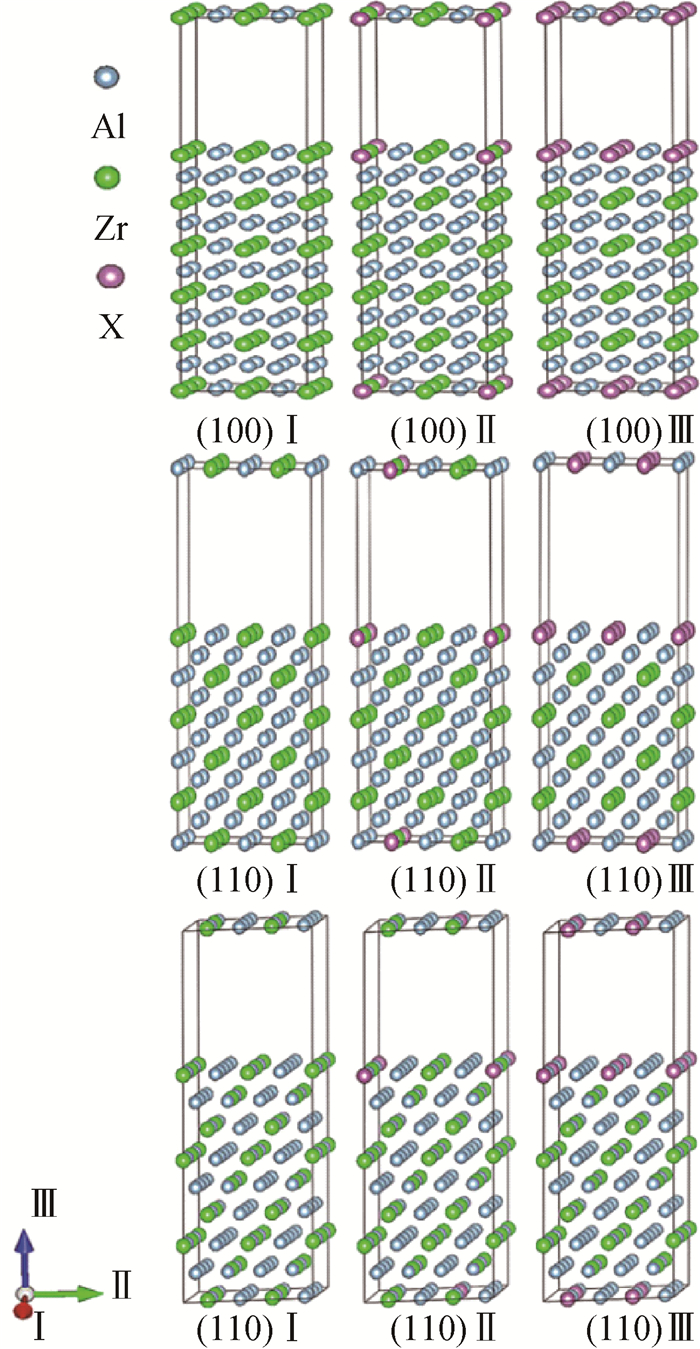
Fig.2 L12-AlxZryXz supercell structure with (100) (110) (111) three different surfaces Ⅰ, Ⅱ and Ⅲ the conditions before doping, doping of X atom with low concentration and doping of X atom with high concentration respectively (Each supercell with a vacuum layer of 1.2 nm.)
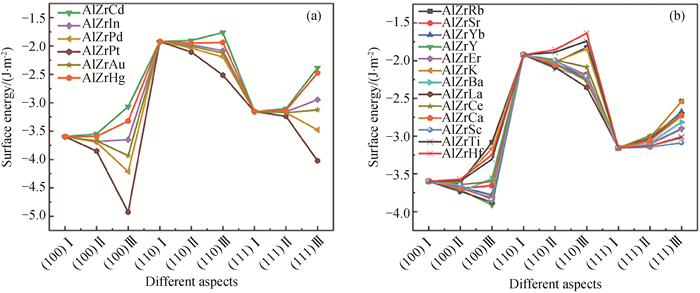
Fig.3 Surface energy of L12-AlxZryXz structures under different doping conditions (a) the Al sites in the L12-Al3Zr precipitated phase by X atoms; (b) the Zr sites in the L12-Al3Zr precipitated phase by X atoms
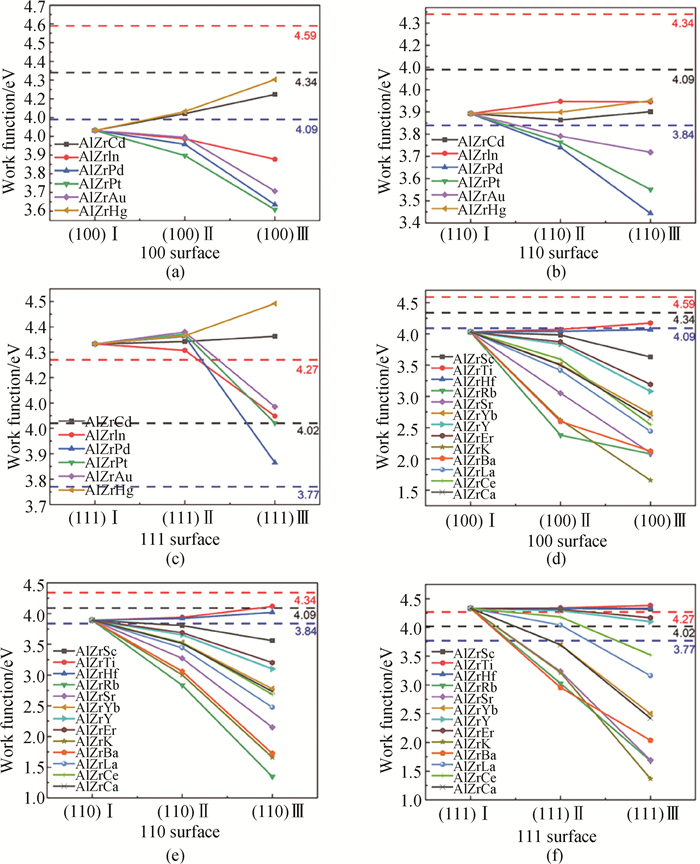
Fig.4 Work function of L12-AlxZryXz structure under different doping conditions (a) (100), (b) (110) and (c) (111) surfaces of the L12-AlxZryXz structure (X atoms occupy the Al sites in the L12-Al3Zr precipitated phase.); (d) (100), (e) (110) and (f) (111) surfaces of the L12-AlxZryXz structure (X atoms occupy the Zr sites in the L12-Al3Zr precipitated phase. The black dashed line represents the work function value of the corresponding surface of Al matrix in the figure, and the red/blue dashed line respectively represents the reference line higher than/lower than the black dashed line work function of 250 meV.)
| atom | electronegativity[ | atom | electronegativity[ |
| In | 1.49 | Er | 1.11 |
| Al | 1.47 | Y | 1.11 |
| Cd | 1.46 | La | 1.08 |
| Hg | 1.44 | Yb | 1.06 |
| Pt | 1.44 | Ce | 1.06 |
| Au | 1.42 | Ca | 1.04 |
| Pd | 1.35 | Sr | 0.99 |
| Ti | 1.32 | Ba | 0.97 |
| Hf | 1.23 | K | 0.91 |
| Zr | 1.22 | Rb | 0.89 |
| Sc | 1.20 |
Table 2 Electronegativity of different atoms
| atom | electronegativity[ | atom | electronegativity[ |
| In | 1.49 | Er | 1.11 |
| Al | 1.47 | Y | 1.11 |
| Cd | 1.46 | La | 1.08 |
| Hg | 1.44 | Yb | 1.06 |
| Pt | 1.44 | Ce | 1.06 |
| Au | 1.42 | Ca | 1.04 |
| Pd | 1.35 | Sr | 0.99 |
| Ti | 1.32 | Ba | 0.97 |
| Hf | 1.23 | K | 0.91 |
| Zr | 1.22 | Rb | 0.89 |
| Sc | 1.20 |
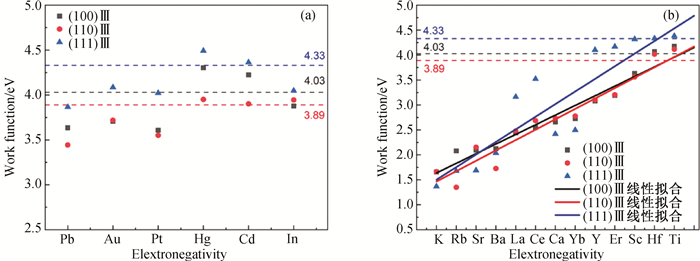
Fig.5 Relation between the Ⅲ surface work function of L12-AlxZryXz structure and electronegativity of doping atoms (The electronegativity of doping atoms gradually increases from left to right, where the black/red/blue dashed lines represent the surface work function values of (100)Ⅲ, (110)Ⅲ and (111)Ⅲ of L12-Al3Zr precipitated phases, respectively.) (a) the Al sites in the L12-Al3Zr precipitated phase by X atoms; (b) the Zr sites in the L12-Al3Zr precipitated phase by X atoms
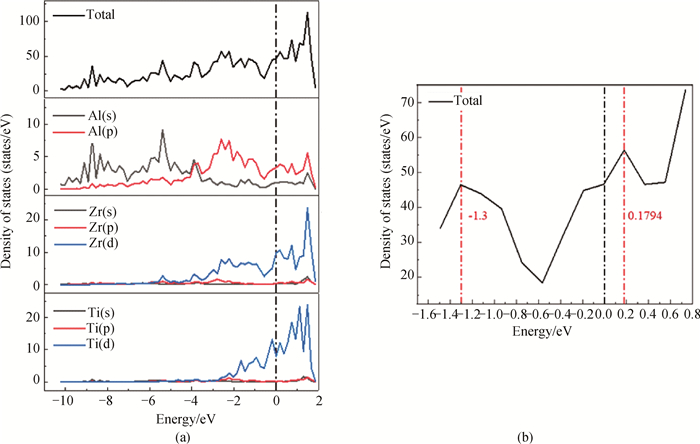
Fig.6 Total density of states (TDOS) and partial density of states (PDOS) on the (100)Ⅲ surface of L12-AlxZryYz precipitated phase (a) (100)Ⅲ surface structure of L12-Al-Zr-Y precipitated phase; (b) enlarged views of the pseudogap
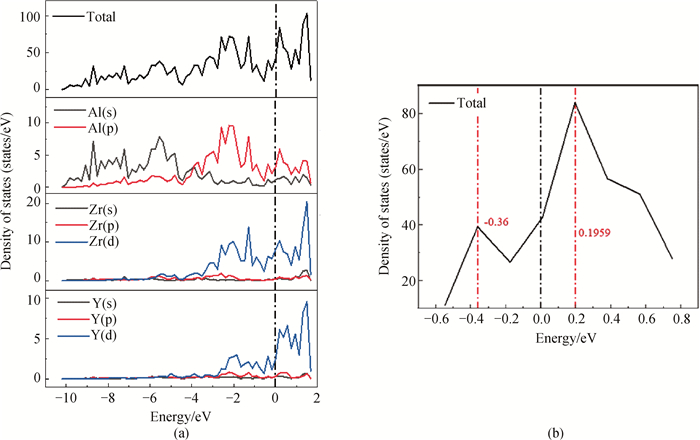
Fig.7 Total density of states (TDOS) and partial density of states (PDOS) on the (100)Ⅲ surface of L12-AlxZryTiz precipitated phase (a) (100)Ⅲ surface structure of L12-Al-Zr-Ti precipitated phase; (b) enlarged views of the pseudogap
| 1 |
WILLIAMS J C , STARKE Jr E A . Progress in structural materials for aerospace systems[J]. Acta Materialia, 2003, 51 (19): 5775- 5799.
DOI |
| 2 | HUDA Z , EDI P . Materials selection in design of structures and engines of supersonic aircrafts: A review[J]. Materials and Design, 2013, 46 (4): 552- 560. |
| 3 |
BIRBILIS N , BUCHHEIT R G . Electrochemical characteristics of intermetallic phases in aluminum alloys an experimental survey and discussion[J]. J Electrochem Soc, 2005, 152 (4): B140- B151.
DOI |
| 4 | CHEN C T , CONG S , CHEN H F , et al. First-principles study of electronic structure and optical properties of Bi doped ZnO[J]. Chinese Journal of Computational Physics, 2018, 35 (6): 720- 728. |
| 5 | ZHANG X Y , FENG L . Electronic structure and magnetic properties of carbon doped Mn3Ge[J]. Chinese Journal of Computational Physics, 2019, 36 (6): 742- 748. |
| 6 | GAO H , YANG Z F , ZHAO J F , et al. Effect of Nb, Sn, Cu, Fe and Cr on Zr (0001) surface nodular corrosion resistence: First-principles study[J]. Chinese Journal of Computational Physics, 2022, 39 (1): 101- 108. |
| 7 | 王健. 电子功函数的计算及其在材料表面电化学问题研究中的应用[D]. 合肥: 中国科学技术大学, 2016. |
| 8 | LI J F , ZIQIAO Z , NA J , et al. Localized corrosion mechanism of 2×××-series Al alloy containing S(Al2CuMg) and θ'(Al2Cu) precipitates in 4.0% NaCl solution at pH 6.1[J]. Materials Chemistry and Physics, 2005, 91 (2/3): 325- 329. |
| 9 | 王晓欢, 张胜寒, 狄杰. 金属电偶腐蚀影响因素研究综述[J]. 广东化工, 2022, 49 (19): 142- 147. |
| 10 | 张国英, 张辉, 方戈亮, 等. Bi, Sb合金化对AZ91镁合金组织、性能影响机理研究[J]. 物理学报, 2005, 54, 5288- 5292. |
| 11 | 张国英, 张辉, 赵子夫, 等. 杂质对镁合金耐蚀性影响的电子理论研究[J]. 物理学报, 2006, 55 (5): 2439- 2443. |
| 12 |
张国英, 张辉, 方戈亮, 等. Al-Zn-Mg-Cu系铝合金中不同区域电子结构及应力腐蚀机理分析[J]. 金属学报, 2009, 45 (6): 687- 691.
DOI |
| 13 |
LI W , LI D Y . On the correlation between surface roughness and work function in copper[J]. J Chem Phys, 2005, 122 (6): 064708.
DOI |
| 14 |
LI D Y , LI W . Electron work function: A parameter sensitive to the adhesion behavior of crystallographic surfaces[J]. Appl Phys Lett, 2001, 79 (26): 4337- 4338.
DOI |
| 15 |
ALCHAGIROV A B , ALCHAGIROV B B , SIZHAZHEV T A , et al. Work function of sodium-rubidium alloys[J]. Russ J Electrochem, 2004, 40 (1): 102- 104.
DOI |
| 16 | 王铀, 王亮. 电子功函数在材料磨损及腐蚀方面的研究进展[J]. 中国材料科技与装备, 2011, 7 (5): 1- 5. |
| 17 | 王健, 王邵青. 铝合金表面电偶腐蚀与电子功函数的关系[J]. 物理化学学报, 2014, 30 (3): 551- 558. |
| 18 | THIERRY D , LEBALLEUR C , LARCHÉ N . Galvanic series in seawater as a function of temperature, oxygen content, and chlorination[J]. Corrosion, 2017, 74 (2): 147- 152. |
| 19 | 陈兴伟, 吴建华, 王佳, 等. 电偶腐蚀影响因素研究进展[J]. 腐蚀科学与防护技术, 2010, 22 (4): 363- 366. |
| 20 | 薪魏, 董超芳, 徐奥妮, 等. 金属腐蚀的多尺度计算模拟研究进展[J]. 中国材料进展, 2018, 37 (1): 1- 8. |
| 21 |
ZHANG C , WANG J , LI X , et al. Rapid screening alloying elements for improved corrosion resistance on the Mg(0001) surface using first principles calculations[J]. Phys Chem Chem Phys, 2021, 23 (47): 26887- 26901.
DOI |
| 22 | KNIPLING K E. Development of a nanoscale precipitation-strengthened creep-resistant aluminum alloy containing trialuminide precipitates[D]. Xi'an: Northwestern University, 2006. |
| 23 | 张永志. Al-Zr-RE(Yb_Y)合金中L12结构相的时效析出机制与作用[D]. 上海: 上海交通大学, 2015. |
| 24 |
姜慧丽, 陈送义, 陈康华, 等. Zr质量分数对7003铝合金组织与腐蚀性能的影响[J]. 中南大学学报, 2017, 48 (10): 2614- 2621.
DOI |
| 25 |
HE Y D , ZHANG X M , YOU J H . Effect of minor Sc and Zr on microstructure and mechanical properties of Al-Zn-Mg-Cu alloy[J]. Trans Nonferrous Met Soc China, 2006, 16 (5): 1228- 1235.
DOI |
| 26 | YU K , LI W X , LI S R , et al. Mechanical properties and microstructure of aluminum alloy 2618 with Al3(Sc, Zr) phases[J]. Mater Sci Eng A, 2004, 368 (1/2): 88- 93. |
| 27 | LI J H , WIESSNER M , ALBU M , et al. Correlative characterization of primary Al3(Sc, Zr) phase in an Al-Zn-Mg based alloy[J]. Mater Charact, 2015, 102 (Apr.): 62- 70. |
| 28 | FANG H C , LUO F H , CHEN K H . Effect of intermetallic phases and recrystallization on the corrosion and fracture behavior of an Al-Zn-Mg-Cu-Zr-Yb-Cr alloy[J]. Mater Sci Eng A, 2017, 684 (Jan.): 480- 490. |
| 29 | 张文毓. 电偶腐蚀与防护的研究进展[J]. 全面腐蚀控制, 2018, 32 (12): 52- 56. |
| 30 | 肖毅. 异种金属的电偶腐蚀行为及防护技术研究[D]. 北京: 华北电力大学, 2006. |
| 31 | ZHANG F , ÖRNEK C , NILSSON J O , et al. Anodisation of aluminium alloy AA7075-influence of intermetallic particles on anodic oxide growth[J]. Corros Sci, 2020, 164, 1- 34. |
| 32 | AO M , LIU H M , DONG C F , et al. Degradation mechanism of 6063 aluminium matrix composite reinforced with TiC and Al2O3 particles[J]. J Alloys Compd, 2021, 859 (Apr.): 157838. |
| 33 | ZUO E , DOU X , CHEN Y , et al. Electronic work function, surface energy and electronic properties of binary Mg-Y and Mg-Al alloys: A DFT study[J]. Surface Science, 2021, 712 (9): 121880. |
| 34 | FANG J X , LU D . Solid state physics[M]. Shanghai: Shanghai Science and Technology Press, 1980. |
| 35 |
ZHOU P , ZHOU C , GONG H R . Chlorine adsorption on Mg, Ca, and MgCa surfaces[J]. Mater Sci Eng C, 2013, 33 (7): 3826- 3831.
DOI |
| 36 |
YUWONO J A , BIRBILIS N , WILLIAMS K S , et al. Electrochemical stability of magnesium surfaces in an aqueous environment[J]. J Phys Chem C, 2016, 120 (47): 26922- 26933.
DOI |
| 37 | LIU Y , HUANG Y C , XIAO Z B , et al. Study of adsorption of hydrogen on Al, Cu, Mg, Ti surfaces in Al alloy melt via first principles calculation[J]. Metals, 2017, 7 (1): 1- 9. |
| 38 | 陈伟, 李云阔, 李耀, 等. 钢中NbC夹杂粒子表面性质的第一性原理[J]. 材料与冶金学报, 2022, 21 (6): 422- 427. |
| 39 | 韩星, 王健, 何志军, 等. 稀土四硼化物表面性质的第一性原理研究[J]. 辽宁科技大学学报, 2022, 45 (3): 216- 220. |
| 40 |
GAULT B , CUI X Y , MOODY M P , et al. Atom probe microscopy investigation of Mg site occupancy within δ' precipitates in an Al-Mg-Li alloy[J]. Scr Mater, 2012, 66 (11): 903- 906.
DOI |
| 41 |
KRESSE G , FURTHMÜLLER J . Efficiency of ab-initio total energy calculations for metals and semiconductors using a plane-wave basis set[J]. Comput Mater Sci, 1996, 6 (1): 15- 50.
DOI |
| 42 |
KRESSE G , HAFNER J . Norm-conserving and ultrasoft pseudopotentials for first-row and transition elements[J]. J Phys: Condens Matter, 1994, 6 (40): 8245- 8257.
DOI |
| 43 |
GONZE X . Towards a potential-based conjugate gradient algorithm for order-N self-consistent total energy calculations[J]. Phys Rev B, 1996, 54 (7): 4383- 4386.
DOI |
| 44 |
FEYNMAN R P . Forces in molecules[J]. Phys Rev, 1939, 56 (4): 340- 343.
DOI |
| 45 | PAN R K , WANG H C , SHI T T , et al. Thermal properties and thermoelasticity of L12 ordered Al3RE (RE=Er, Tm, Yb, Lu) phases: A first-principles study[J]. Mater Des, 2016, 102 (15): 100- 105. |
| 46 |
MONKHORST H J , PACK J D . Special points for Brillouin-zone integrations[J]. Phys Rev B, 1976, 13 (12): 5188- 5192.
DOI |
| 47 |
GREPSTAD J K , GARTLAND P O , SLAGSVOLD B J . Anisotropic work function of clean and smooth low-index faces of aluminium[J]. Surface Science, 1976, 57 (1): 348- 362.
DOI |
| 48 | SINGH-MILLER N E , MARZARI N . Surface energies, work functions, and surface relaxations of low-index metallic surfaces from first principles[J]. Phys Rev B, 2009, 80 (23): 1- 9. |
| 49 |
SMOLUCHOWSKI R . Anisotropy of the electronic work function of metals[J]. Phys Rev, 1941, 60 (9): 661- 674.
DOI |
| 50 |
LI H Y , LI D W , ZHU Z X , et al. Grain refinement mechanism of as-cast aluminum by hafnium[J]. Transactions of Nonferrous Metals Society of China, 2016, 26 (12): 3059- 3069.
DOI |
| 51 | 王郁, 王俊升, 薛程鹏, 等. 微合金化对铝合金高温析出相影响的研究进展[J]. 航空制造技术, 2021, 64 (15): 68- 77. |
| 52 | 王旭东, 聂祚仁, 林双平, 等. Er对Al-Zn-Mg-Cu合金抗腐蚀性能的影响[J]. 特种铸造及有色合金, 2009, 29 (1): 76- 78. |
| 53 | 韩剑, 戴起勋, 李桂荣, 等. 稀土钇对7055铝合金铸态组织的影响[J]. 材料工程, 2009, (4): 67- 70. |
| 54 | 赖人铭, 计熊, 赵国忠, 等. 稀土元素La对6063铝合金组织和性能的影响[J]. 轻合金加工技术, 2007, 35 (10): 28- 32. |
| 55 | 孔敏. 第一性原理研究铝合金中间相的腐蚀机理[D]. 桂林: 桂林理工大学, 2020. |
| 56 | 袁汉杰. 电负性的研究-原子的电负性[J]. 化学学报, 1964, (3): 103- 109. |
| 57 | 张敏刚, 梁志彬, 闫时建, 等. Nb金化Mg2Ni及其氢化物能量和电子结构的第一性原理研究[J]. 稀有金属材料与工程, 2015, 44 (2): 386- 390. |
| No related articles found! |
| Viewed | ||||||
|
Full text |
|
|||||
|
Abstract |
|
|||||
Copyright © Chinese Journal of Computational Physics
E-mail: jswl@iapcm.ac.cn
Supported by Beijing Magtech Co., Ltd.
