Chinese Journal of Computational Physics ›› 2022, Vol. 39 ›› Issue (3): 335-340.DOI: 10.19596/j.cnki.1001-246x.8402
• Research Reports • Previous Articles Next Articles
Jin LIU( ), Enqi SUN, Xuexian YANG*(
), Enqi SUN, Xuexian YANG*( ), Ling ZHU, Yonggang HUANG
), Ling ZHU, Yonggang HUANG
Received:2021-05-24
Online:2022-05-25
Published:2022-09-02
Contact:
Xuexian YANG
Jin LIU, Enqi SUN, Xuexian YANG, Ling ZHU, Yonggang HUANG. Temperature Dependence of Bond Length, Debye Temperature and Thermal Expansion Coefficient of Alkali Halides[J]. Chinese Journal of Computational Physics, 2022, 39(3): 335-340.
Add to citation manager EndNote|Ris|BibTeX
URL: http://www.cjcp.org.cn/EN/10.19596/j.cnki.1001-246x.8402
| Material | α(T) | l(T) | References | |||||||
| θD(0)/K | A1(r) | θD(0)/K | A1(r) | l0/nm | θD(0)/K[ | Tm/K[ | EB(0)/eV[ | |||
| LiF | 742 | 0.977 | 735 | 0.979 | 0.200 03 | 735, 751 | 1 143 | 5.42 | ||
| LiCl | 429 | 1.153 | 429 | 1.153 | 0.254 97 | 429, 530 | 887 | 4.43 | ||
| LiBr | 321 | 1.119 | 300 | 1.119 | 0.272 39 | 274, 465 | 820 | 4.26 | ||
| LiI | 310 | 1.022 | 290 | 1.014 | 0.297 06 | 410 | 719 | 3.96 | ||
| NaF | 488 | 1.401 | 488 | 1.414 | 0.230 45 | 488, 470 | 1 205 | 4.81 | ||
| NaCl | 350 | 1.438 | 321 | 1.430 | 0.279 86 | 321, 320 | 1 050 | 4.09 | ||
| NaBr | 270 | 1.489 | 270 | 1.489 | 0.296 28 | 225, 264 | 1 028 | 3.86 | ||
| NaI | 235 | 1.518 | 235 | 1.493 | 0.320 49 | 164, 230 | 928 | 3.66 | ||
Table 1 Bond length, melting point, atomic cohesive energy, Debye temperature and rigidity factor of alkali halides
| Material | α(T) | l(T) | References | |||||||
| θD(0)/K | A1(r) | θD(0)/K | A1(r) | l0/nm | θD(0)/K[ | Tm/K[ | EB(0)/eV[ | |||
| LiF | 742 | 0.977 | 735 | 0.979 | 0.200 03 | 735, 751 | 1 143 | 5.42 | ||
| LiCl | 429 | 1.153 | 429 | 1.153 | 0.254 97 | 429, 530 | 887 | 4.43 | ||
| LiBr | 321 | 1.119 | 300 | 1.119 | 0.272 39 | 274, 465 | 820 | 4.26 | ||
| LiI | 310 | 1.022 | 290 | 1.014 | 0.297 06 | 410 | 719 | 3.96 | ||
| NaF | 488 | 1.401 | 488 | 1.414 | 0.230 45 | 488, 470 | 1 205 | 4.81 | ||
| NaCl | 350 | 1.438 | 321 | 1.430 | 0.279 86 | 321, 320 | 1 050 | 4.09 | ||
| NaBr | 270 | 1.489 | 270 | 1.489 | 0.296 28 | 225, 264 | 1 028 | 3.86 | ||
| NaI | 235 | 1.518 | 235 | 1.493 | 0.320 49 | 164, 230 | 928 | 3.66 | ||

Fig.1 Theoretical curves (lines) and experimental data[27-28] (scattered dots) of temperature dependence of thermal expansion coefficients of (a) Li and (b) Na group alkali halides
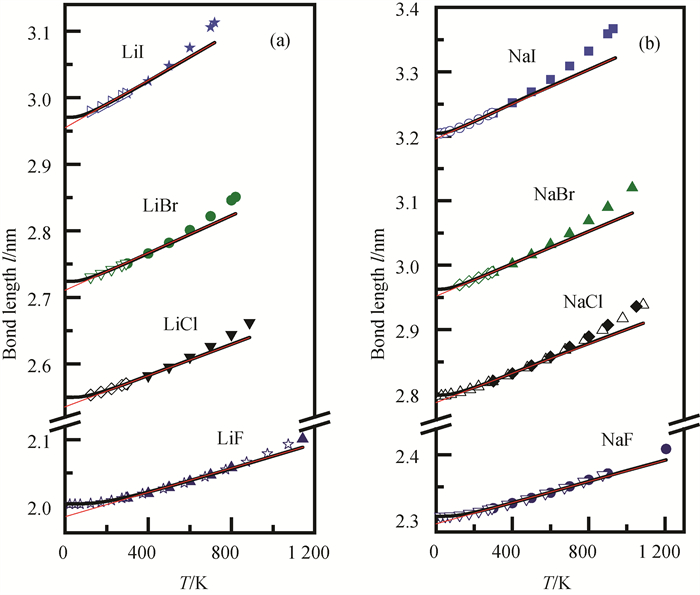
Fig.2 Theoretical values (black lines) and experimental data[27, 33-34] (scattered dots) of temperature dependence of bond length of (a) Li group and (b) Na group alkali halides (The red lines denote tangents of theoretical values over the linear range of high temperature.)
| 1 |
任国浩, 杨帆. 卤化物闪烁晶体的研究历史和现状[J]. 中国科学: 技术科学, 2017, 47 (11): 1149- 1164.
|
| 2 |
王秀芳, 杨金科, 黄多辉. 高温高压下LiF热力学性质的第一性原理计算[J]. 材料导报B: 研究篇, 2013, 27 (5): 140- 156.
|
| 3 |
范志达, 王强涛, 尹利君, 等. 氟化锂晶体的研究进展[J]. 硅酸盐通报, 2010, 29 (4): 893- 897.
|
| 4 |
DOI |
| 5 |
DOI |
| 6 |
DOI |
| 7 |
DOI |
| 8 |
郭蕾, 张永春. 碘化钠晶体应用综述[J]. 硅酸盐通报, 2015, 34 (S1): 293- 297.
|
| 9 |
韩善彪, 吕雪艳, 任建保, 等. 航空辐射监测发展现状和建议[J]. 核电子学与探测技术, 2019, 39 (1): 111- 117.
DOI |
| 10 |
|
| 11 |
|
| 12 |
|
| 13 |
|
| 14 |
DOI |
| 15 |
DOI |
| 16 |
|
| 17 |
|
| 18 |
|
| 19 |
|
| 20 |
|
| 21 |
DOI |
| 22 |
DOI |
| 23 |
DOI |
| 24 |
|
| 25 |
DOI |
| 26 |
DOI |
| 27 |
|
| 28 |
DOI |
| 29 |
DOI |
| 30 |
DOI |
| 31 |
DOI |
| 32 |
|
| 33 |
DOI |
| 34 |
DOI |
| 35 |
DOI |
| [1] | ZONG Liang, XU Xiaojing, ZHOU Hai. Molecular Dynamics Simulation of Tension Deformation in Monocrystalline β-SiC Bulk [J]. CHINESE JOURNAL OF COMPUTATIONAL PHYSICS, 2010, 27(6): 898-904. |
| [2] | CHEN Wen, ZHOU Jing, XU Qing, LI Yue-ming, Sun Hua-jun, MIN Xin-min. Electronic Structure and Poling Characteristics of Na1/2Bi1/2TiO3 System [J]. CHINESE JOURNAL OF COMPUTATIONAL PHYSICS, 2004, 21(6): 543-546. |
| [3] | MIN Xin-min, ZHU Lei, XING Xue-lingnd. Electronic Structure of Ca3Co2O6 and Ni-doped Ones [J]. CHINESE JOURNAL OF COMPUTATIONAL PHYSICS, 2004, 21(3): 373-376. |
| [4] | Li Beixing, Feng Xiuji, Min Xinmin. STUDIES ON QUANTUM CHEMISTRY CALCULATIONS OF STRUCTURE AND PROPERTIES OF ALUMINATE CEMENT MINERALS [J]. CHINESE JOURNAL OF COMPUTATIONAL PHYSICS, 1998, 15(6): 757-760. |
| Viewed | ||||||
|
Full text |
|
|||||
|
Abstract |
|
|||||
Copyright © Chinese Journal of Computational Physics
E-mail: jswl@iapcm.ac.cn
Supported by Beijing Magtech Co., Ltd.
