计算物理 ›› 2023, Vol. 40 ›› Issue (6): 699-711.DOI: 10.19596/j.cnki.1001-246x.8678
收稿日期:2022-12-12
出版日期:2023-11-25
发布日期:2024-01-22
通讯作者:
刘凌虹
作者简介:张庆洲, 男, 硕士研究生, 研究方向为合金元素对铝合金耐蚀性影响, E-mail: 1711812275@qq.com
基金资助:
Qingzhou ZHANG1,2( ), Dawei FAN1,2, Linghong LIU1,2,*(
), Dawei FAN1,2, Linghong LIU1,2,*( )
)
Received:2022-12-12
Online:2023-11-25
Published:2024-01-22
Contact:
Linghong LIU
摘要:
本文在AlZr二元合金的基础上, 利用第一性原理计算方法, 全面计算19种L12-AlxZryXz(X=Pd、Pt、Au、K、Rb、Sr、Ba、Ca、Yb、La、Ce、Y、Er、Sc、Zr、Ti、Cd、Hf、In)三元析出相的(100)、(110)和(111)三种晶面作为表面时的功函数, 并分析其与掺杂原子电负性之间的关系, 从电子层面阐明掺杂原子对L12型Al-Zr-X三元铝合金表面电偶腐蚀性能影响的根本原因。通过计算发现不同掺杂晶面暴露为表面时, 由于功函数各异, 与基体的本征电位差也各不相同。Hg、Cd、Zr、Ti和Hf等掺杂原子能增加析出相(100)表面的功函数, Hg、Cd、In、Ti和Hf等原子能增加析出相(110)面的功函数, 而Pd、Pt、Au、In、Sc、Rb、Sr、Yb、Y、Er、K、Ba、La、Ce和Ca原子能降低(111)面的功函数, 这些均导致析出相与铝基体的本征电位差进一步减小。此外, 结果揭示了掺杂原子电负性与其化合物功函数之间的线性正相关规律。相较而言, 电负性与Al接近且替代Al的In、Cd和Hg原子, 以及电负性与Zr接近且替代Zr的Ti和Hf原子对析出相功函数影响较小, 且其化合物与铝基体的电势差较小, 对提升材料抗腐蚀能力有益, 其他掺杂原子则对析出相功函数有较大影响。研究解释了部分耐蚀性实验结果, 为优化合金成分设计、提升铝合金材料耐腐蚀性提供了理论参考。
中图分类号:
张庆洲, 范大伟, 刘凌虹. 合金原子对L12型Al-Zr-X三元铝合金表面电偶腐蚀影响的第一性原理研究[J]. 计算物理, 2023, 40(6): 699-711.
Qingzhou ZHANG, Dawei FAN, Linghong LIU. First-principles Study on the Influence of Alloying Elements on Galvanic Corrosion of Ternary L12-Al-Zr-X Aluminum Alloys Surface[J]. Chinese Journal of Computational Physics, 2023, 40(6): 699-711.
| Surface | W/eV | Wexp/eV | Esurf/(J·m-2) | ||
| (100) | 4.34 | 4.30[ | 4.41[ | 1.03 | 0.96[ |
| (110) | 4.09 | 4.09[ | 4.28[ | 1.04 | 1.02[ |
| (111) | 4.02 | 4.02[ | 4.24[ | 0.84 | 0.73[ |
表1 铝基体不同表面的功函数(W)和表面能(Esurf)
Table 1 Work function (W) and surface energy (Esurf) of different surfaces of Al matrix
| Surface | W/eV | Wexp/eV | Esurf/(J·m-2) | ||
| (100) | 4.34 | 4.30[ | 4.41[ | 1.03 | 0.96[ |
| (110) | 4.09 | 4.09[ | 4.28[ | 1.04 | 1.02[ |
| (111) | 4.02 | 4.02[ | 4.24[ | 0.84 | 0.73[ |
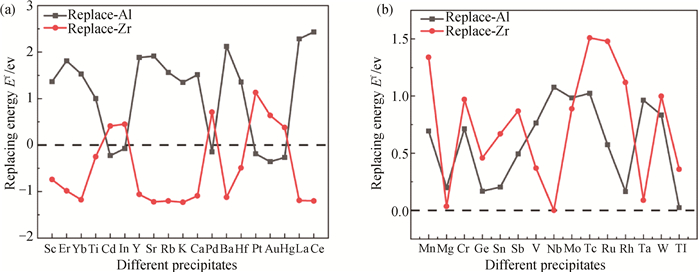
图1 X原子取代L12-Al3Zr二元析出相中Al/Zr位的替换形成焓Ef(虚线表示Ef=0) (a) X原子取代Al/Zr原子位置;(b) X原子未取代Al/Zr原子位置
Fig.1 Substitution formation enthalpy Ef of the Al/Zr sites in the precipitated phase of L12-Al3Zr binary by X atoms (the dotted line indicates Ef=0) (a) the substitution of Al/Zr atoms by X atom; (b) the substitution of Al/Zr atoms not by X atoms
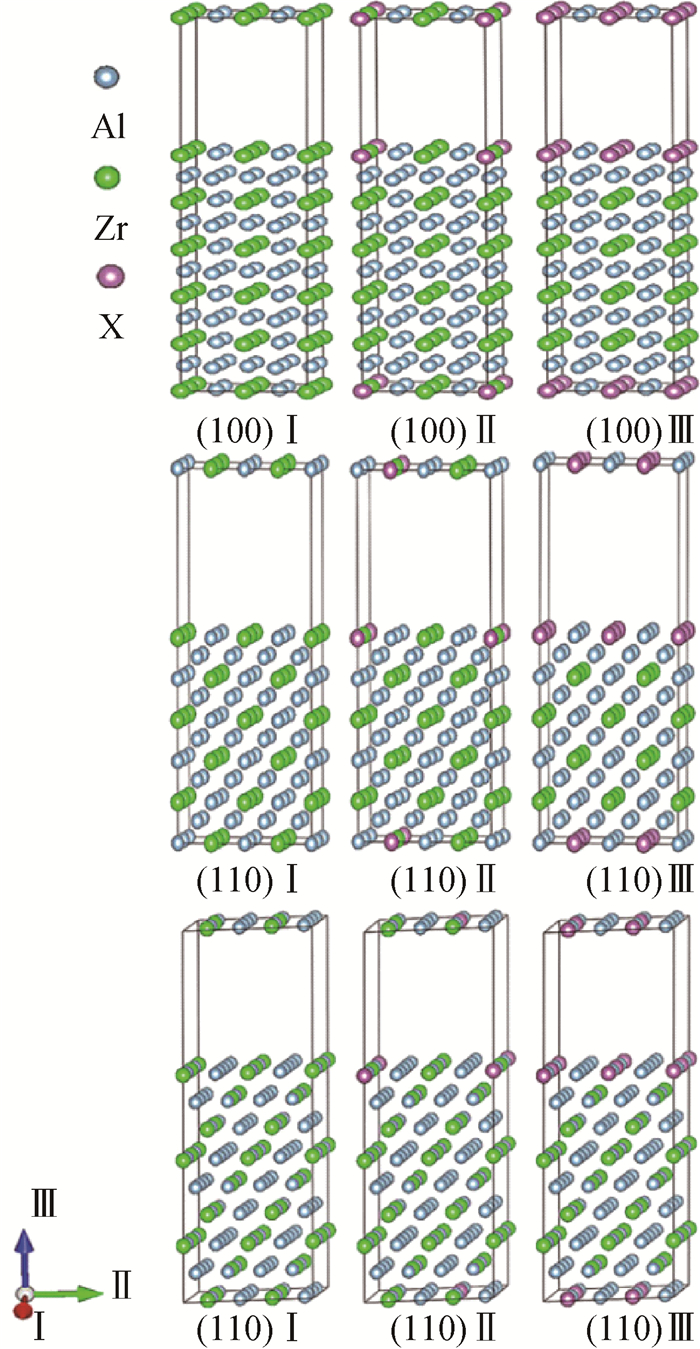
图2 包含(100)、(110)和(111)3个不同表面的L12-AlxZryXz超胞模型(Ⅰ、Ⅱ、Ⅲ分别表示掺杂前、X原子低浓度掺杂和X原子高浓度掺杂的情况。每个超胞都包含一个1.2 nm的真空层。)
Fig.2 L12-AlxZryXz supercell structure with (100) (110) (111) three different surfaces Ⅰ, Ⅱ and Ⅲ the conditions before doping, doping of X atom with low concentration and doping of X atom with high concentration respectively (Each supercell with a vacuum layer of 1.2 nm.)
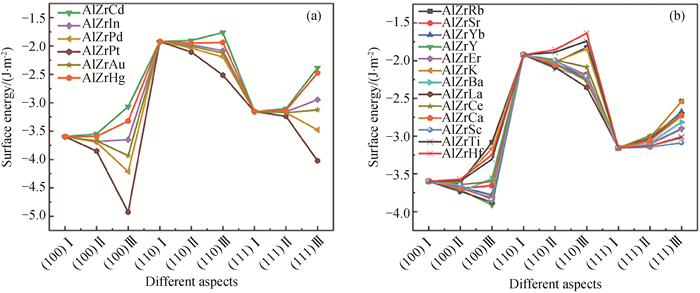
图3 不同掺杂条件下L12-AlxZryXz结构的表面能(a) X原子占据L12-Al3Zr析出相的Al位;(b) X原子占据L12-Al3Zr析出相的Zr位
Fig.3 Surface energy of L12-AlxZryXz structures under different doping conditions (a) the Al sites in the L12-Al3Zr precipitated phase by X atoms; (b) the Zr sites in the L12-Al3Zr precipitated phase by X atoms
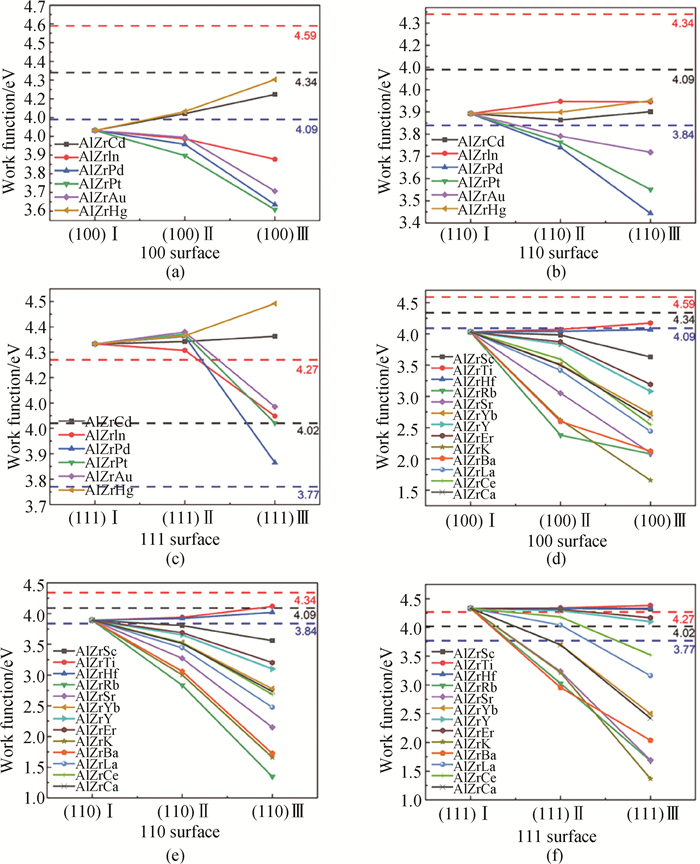
图4 不同掺杂条件下L12-AlxZryXz结构的功函数(a)、(b)和(c)分别为L12-AlxZryXz结构的(100)、(110)和(111)面(X原子在L12-Al3Zr析出相中占据Al位。);(d)、(e)和(f)分别为L12-AlxZryXz结构的(100)、(110)和(111)面(X原子在L12-Al3Zr析出相中占据Zr位。黑色虚线为图中Al矩阵对应曲面的功函数值,红色/蓝色虚线分别为高于/低于黑色虚线功函数250 meV的参考线。)
Fig.4 Work function of L12-AlxZryXz structure under different doping conditions (a) (100), (b) (110) and (c) (111) surfaces of the L12-AlxZryXz structure (X atoms occupy the Al sites in the L12-Al3Zr precipitated phase.); (d) (100), (e) (110) and (f) (111) surfaces of the L12-AlxZryXz structure (X atoms occupy the Zr sites in the L12-Al3Zr precipitated phase. The black dashed line represents the work function value of the corresponding surface of Al matrix in the figure, and the red/blue dashed line respectively represents the reference line higher than/lower than the black dashed line work function of 250 meV.)
| atom | electronegativity[ | atom | electronegativity[ |
| In | 1.49 | Er | 1.11 |
| Al | 1.47 | Y | 1.11 |
| Cd | 1.46 | La | 1.08 |
| Hg | 1.44 | Yb | 1.06 |
| Pt | 1.44 | Ce | 1.06 |
| Au | 1.42 | Ca | 1.04 |
| Pd | 1.35 | Sr | 0.99 |
| Ti | 1.32 | Ba | 0.97 |
| Hf | 1.23 | K | 0.91 |
| Zr | 1.22 | Rb | 0.89 |
| Sc | 1.20 |
表2 不同原子的电负性
Table 2 Electronegativity of different atoms
| atom | electronegativity[ | atom | electronegativity[ |
| In | 1.49 | Er | 1.11 |
| Al | 1.47 | Y | 1.11 |
| Cd | 1.46 | La | 1.08 |
| Hg | 1.44 | Yb | 1.06 |
| Pt | 1.44 | Ce | 1.06 |
| Au | 1.42 | Ca | 1.04 |
| Pd | 1.35 | Sr | 0.99 |
| Ti | 1.32 | Ba | 0.97 |
| Hf | 1.23 | K | 0.91 |
| Zr | 1.22 | Rb | 0.89 |
| Sc | 1.20 |
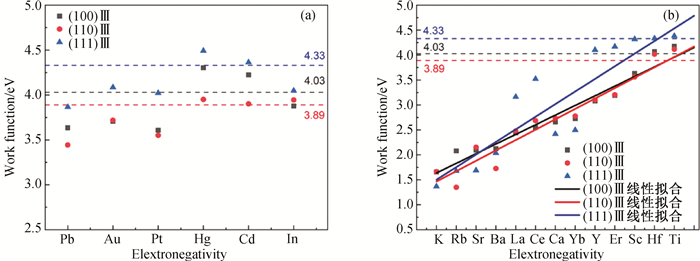
图5 L12-AlxZryXz结构Ⅲ表面功函数与掺杂原子电负性关系(掺杂原子的电负性从左到右逐渐增大,其中黑色/红色/蓝色虚线分别代表L12-Al3Zr析出相(100)Ⅲ、(110)Ⅲ和(111)Ⅲ的表面功函数值。) (a) X原子占据L12-Al3Zr析出相的Al位;(b) X原子占据L12-Al3Zr析出相的Zr位。
Fig.5 Relation between the Ⅲ surface work function of L12-AlxZryXz structure and electronegativity of doping atoms (The electronegativity of doping atoms gradually increases from left to right, where the black/red/blue dashed lines represent the surface work function values of (100)Ⅲ, (110)Ⅲ and (111)Ⅲ of L12-Al3Zr precipitated phases, respectively.) (a) the Al sites in the L12-Al3Zr precipitated phase by X atoms; (b) the Zr sites in the L12-Al3Zr precipitated phase by X atoms
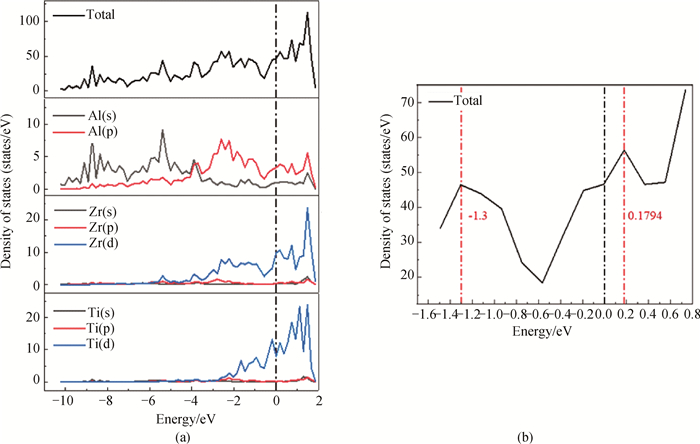
图6 L12-AlxZryYz析出相(100)Ⅲ表面的总态密度(TDOS)和部分态密度(PDOS)(a)L12-Al-Zr-Y析出相的(100)Ⅲ表面结构; (b)为赝能隙的放大视图
Fig.6 Total density of states (TDOS) and partial density of states (PDOS) on the (100)Ⅲ surface of L12-AlxZryYz precipitated phase (a) (100)Ⅲ surface structure of L12-Al-Zr-Y precipitated phase; (b) enlarged views of the pseudogap
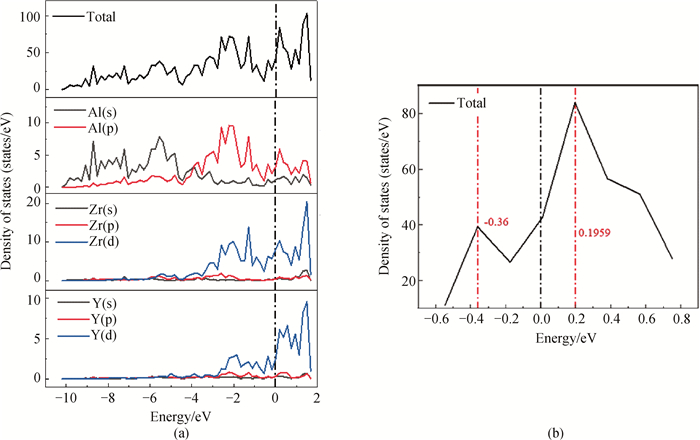
图7 L12-AlxZryTiz析出相(100)Ⅲ表面的总态密度(TDOS)和部分态密度(PDOS)(a)L12-Al-Zr-Ti析出相的(100)Ⅲ表面结构; (b)为赝能隙的放大视图
Fig.7 Total density of states (TDOS) and partial density of states (PDOS) on the (100)Ⅲ surface of L12-AlxZryTiz precipitated phase (a) (100)Ⅲ surface structure of L12-Al-Zr-Ti precipitated phase; (b) enlarged views of the pseudogap
| 1 |
WILLIAMS J C , STARKE Jr E A . Progress in structural materials for aerospace systems[J]. Acta Materialia, 2003, 51 (19): 5775- 5799.
DOI |
| 2 | HUDA Z , EDI P . Materials selection in design of structures and engines of supersonic aircrafts: A review[J]. Materials and Design, 2013, 46 (4): 552- 560. |
| 3 |
BIRBILIS N , BUCHHEIT R G . Electrochemical characteristics of intermetallic phases in aluminum alloys an experimental survey and discussion[J]. J Electrochem Soc, 2005, 152 (4): B140- B151.
DOI |
| 4 | CHEN C T , CONG S , CHEN H F , et al. First-principles study of electronic structure and optical properties of Bi doped ZnO[J]. Chinese Journal of Computational Physics, 2018, 35 (6): 720- 728. |
| 5 | ZHANG X Y , FENG L . Electronic structure and magnetic properties of carbon doped Mn3Ge[J]. Chinese Journal of Computational Physics, 2019, 36 (6): 742- 748. |
| 6 | GAO H , YANG Z F , ZHAO J F , et al. Effect of Nb, Sn, Cu, Fe and Cr on Zr (0001) surface nodular corrosion resistence: First-principles study[J]. Chinese Journal of Computational Physics, 2022, 39 (1): 101- 108. |
| 7 | 王健. 电子功函数的计算及其在材料表面电化学问题研究中的应用[D]. 合肥: 中国科学技术大学, 2016. |
| 8 | LI J F , ZIQIAO Z , NA J , et al. Localized corrosion mechanism of 2×××-series Al alloy containing S(Al2CuMg) and θ'(Al2Cu) precipitates in 4.0% NaCl solution at pH 6.1[J]. Materials Chemistry and Physics, 2005, 91 (2/3): 325- 329. |
| 9 | 王晓欢, 张胜寒, 狄杰. 金属电偶腐蚀影响因素研究综述[J]. 广东化工, 2022, 49 (19): 142- 147. |
| 10 | 张国英, 张辉, 方戈亮, 等. Bi, Sb合金化对AZ91镁合金组织、性能影响机理研究[J]. 物理学报, 2005, 54, 5288- 5292. |
| 11 | 张国英, 张辉, 赵子夫, 等. 杂质对镁合金耐蚀性影响的电子理论研究[J]. 物理学报, 2006, 55 (5): 2439- 2443. |
| 12 |
张国英, 张辉, 方戈亮, 等. Al-Zn-Mg-Cu系铝合金中不同区域电子结构及应力腐蚀机理分析[J]. 金属学报, 2009, 45 (6): 687- 691.
DOI |
| 13 |
LI W , LI D Y . On the correlation between surface roughness and work function in copper[J]. J Chem Phys, 2005, 122 (6): 064708.
DOI |
| 14 |
LI D Y , LI W . Electron work function: A parameter sensitive to the adhesion behavior of crystallographic surfaces[J]. Appl Phys Lett, 2001, 79 (26): 4337- 4338.
DOI |
| 15 |
ALCHAGIROV A B , ALCHAGIROV B B , SIZHAZHEV T A , et al. Work function of sodium-rubidium alloys[J]. Russ J Electrochem, 2004, 40 (1): 102- 104.
DOI |
| 16 | 王铀, 王亮. 电子功函数在材料磨损及腐蚀方面的研究进展[J]. 中国材料科技与装备, 2011, 7 (5): 1- 5. |
| 17 | 王健, 王邵青. 铝合金表面电偶腐蚀与电子功函数的关系[J]. 物理化学学报, 2014, 30 (3): 551- 558. |
| 18 | THIERRY D , LEBALLEUR C , LARCHÉ N . Galvanic series in seawater as a function of temperature, oxygen content, and chlorination[J]. Corrosion, 2017, 74 (2): 147- 152. |
| 19 | 陈兴伟, 吴建华, 王佳, 等. 电偶腐蚀影响因素研究进展[J]. 腐蚀科学与防护技术, 2010, 22 (4): 363- 366. |
| 20 | 薪魏, 董超芳, 徐奥妮, 等. 金属腐蚀的多尺度计算模拟研究进展[J]. 中国材料进展, 2018, 37 (1): 1- 8. |
| 21 |
ZHANG C , WANG J , LI X , et al. Rapid screening alloying elements for improved corrosion resistance on the Mg(0001) surface using first principles calculations[J]. Phys Chem Chem Phys, 2021, 23 (47): 26887- 26901.
DOI |
| 22 | KNIPLING K E. Development of a nanoscale precipitation-strengthened creep-resistant aluminum alloy containing trialuminide precipitates[D]. Xi'an: Northwestern University, 2006. |
| 23 | 张永志. Al-Zr-RE(Yb_Y)合金中L12结构相的时效析出机制与作用[D]. 上海: 上海交通大学, 2015. |
| 24 |
姜慧丽, 陈送义, 陈康华, 等. Zr质量分数对7003铝合金组织与腐蚀性能的影响[J]. 中南大学学报, 2017, 48 (10): 2614- 2621.
DOI |
| 25 |
HE Y D , ZHANG X M , YOU J H . Effect of minor Sc and Zr on microstructure and mechanical properties of Al-Zn-Mg-Cu alloy[J]. Trans Nonferrous Met Soc China, 2006, 16 (5): 1228- 1235.
DOI |
| 26 | YU K , LI W X , LI S R , et al. Mechanical properties and microstructure of aluminum alloy 2618 with Al3(Sc, Zr) phases[J]. Mater Sci Eng A, 2004, 368 (1/2): 88- 93. |
| 27 | LI J H , WIESSNER M , ALBU M , et al. Correlative characterization of primary Al3(Sc, Zr) phase in an Al-Zn-Mg based alloy[J]. Mater Charact, 2015, 102 (Apr.): 62- 70. |
| 28 | FANG H C , LUO F H , CHEN K H . Effect of intermetallic phases and recrystallization on the corrosion and fracture behavior of an Al-Zn-Mg-Cu-Zr-Yb-Cr alloy[J]. Mater Sci Eng A, 2017, 684 (Jan.): 480- 490. |
| 29 | 张文毓. 电偶腐蚀与防护的研究进展[J]. 全面腐蚀控制, 2018, 32 (12): 52- 56. |
| 30 | 肖毅. 异种金属的电偶腐蚀行为及防护技术研究[D]. 北京: 华北电力大学, 2006. |
| 31 | ZHANG F , ÖRNEK C , NILSSON J O , et al. Anodisation of aluminium alloy AA7075-influence of intermetallic particles on anodic oxide growth[J]. Corros Sci, 2020, 164, 1- 34. |
| 32 | AO M , LIU H M , DONG C F , et al. Degradation mechanism of 6063 aluminium matrix composite reinforced with TiC and Al2O3 particles[J]. J Alloys Compd, 2021, 859 (Apr.): 157838. |
| 33 | ZUO E , DOU X , CHEN Y , et al. Electronic work function, surface energy and electronic properties of binary Mg-Y and Mg-Al alloys: A DFT study[J]. Surface Science, 2021, 712 (9): 121880. |
| 34 | FANG J X , LU D . Solid state physics[M]. Shanghai: Shanghai Science and Technology Press, 1980. |
| 35 |
ZHOU P , ZHOU C , GONG H R . Chlorine adsorption on Mg, Ca, and MgCa surfaces[J]. Mater Sci Eng C, 2013, 33 (7): 3826- 3831.
DOI |
| 36 |
YUWONO J A , BIRBILIS N , WILLIAMS K S , et al. Electrochemical stability of magnesium surfaces in an aqueous environment[J]. J Phys Chem C, 2016, 120 (47): 26922- 26933.
DOI |
| 37 | LIU Y , HUANG Y C , XIAO Z B , et al. Study of adsorption of hydrogen on Al, Cu, Mg, Ti surfaces in Al alloy melt via first principles calculation[J]. Metals, 2017, 7 (1): 1- 9. |
| 38 | 陈伟, 李云阔, 李耀, 等. 钢中NbC夹杂粒子表面性质的第一性原理[J]. 材料与冶金学报, 2022, 21 (6): 422- 427. |
| 39 | 韩星, 王健, 何志军, 等. 稀土四硼化物表面性质的第一性原理研究[J]. 辽宁科技大学学报, 2022, 45 (3): 216- 220. |
| 40 |
GAULT B , CUI X Y , MOODY M P , et al. Atom probe microscopy investigation of Mg site occupancy within δ' precipitates in an Al-Mg-Li alloy[J]. Scr Mater, 2012, 66 (11): 903- 906.
DOI |
| 41 |
KRESSE G , FURTHMÜLLER J . Efficiency of ab-initio total energy calculations for metals and semiconductors using a plane-wave basis set[J]. Comput Mater Sci, 1996, 6 (1): 15- 50.
DOI |
| 42 |
KRESSE G , HAFNER J . Norm-conserving and ultrasoft pseudopotentials for first-row and transition elements[J]. J Phys: Condens Matter, 1994, 6 (40): 8245- 8257.
DOI |
| 43 |
GONZE X . Towards a potential-based conjugate gradient algorithm for order-N self-consistent total energy calculations[J]. Phys Rev B, 1996, 54 (7): 4383- 4386.
DOI |
| 44 |
FEYNMAN R P . Forces in molecules[J]. Phys Rev, 1939, 56 (4): 340- 343.
DOI |
| 45 | PAN R K , WANG H C , SHI T T , et al. Thermal properties and thermoelasticity of L12 ordered Al3RE (RE=Er, Tm, Yb, Lu) phases: A first-principles study[J]. Mater Des, 2016, 102 (15): 100- 105. |
| 46 |
MONKHORST H J , PACK J D . Special points for Brillouin-zone integrations[J]. Phys Rev B, 1976, 13 (12): 5188- 5192.
DOI |
| 47 |
GREPSTAD J K , GARTLAND P O , SLAGSVOLD B J . Anisotropic work function of clean and smooth low-index faces of aluminium[J]. Surface Science, 1976, 57 (1): 348- 362.
DOI |
| 48 | SINGH-MILLER N E , MARZARI N . Surface energies, work functions, and surface relaxations of low-index metallic surfaces from first principles[J]. Phys Rev B, 2009, 80 (23): 1- 9. |
| 49 |
SMOLUCHOWSKI R . Anisotropy of the electronic work function of metals[J]. Phys Rev, 1941, 60 (9): 661- 674.
DOI |
| 50 |
LI H Y , LI D W , ZHU Z X , et al. Grain refinement mechanism of as-cast aluminum by hafnium[J]. Transactions of Nonferrous Metals Society of China, 2016, 26 (12): 3059- 3069.
DOI |
| 51 | 王郁, 王俊升, 薛程鹏, 等. 微合金化对铝合金高温析出相影响的研究进展[J]. 航空制造技术, 2021, 64 (15): 68- 77. |
| 52 | 王旭东, 聂祚仁, 林双平, 等. Er对Al-Zn-Mg-Cu合金抗腐蚀性能的影响[J]. 特种铸造及有色合金, 2009, 29 (1): 76- 78. |
| 53 | 韩剑, 戴起勋, 李桂荣, 等. 稀土钇对7055铝合金铸态组织的影响[J]. 材料工程, 2009, (4): 67- 70. |
| 54 | 赖人铭, 计熊, 赵国忠, 等. 稀土元素La对6063铝合金组织和性能的影响[J]. 轻合金加工技术, 2007, 35 (10): 28- 32. |
| 55 | 孔敏. 第一性原理研究铝合金中间相的腐蚀机理[D]. 桂林: 桂林理工大学, 2020. |
| 56 | 袁汉杰. 电负性的研究-原子的电负性[J]. 化学学报, 1964, (3): 103- 109. |
| 57 | 张敏刚, 梁志彬, 闫时建, 等. Nb金化Mg2Ni及其氢化物能量和电子结构的第一性原理研究[J]. 稀有金属材料与工程, 2015, 44 (2): 386- 390. |
| [1] | 程翔, 冯军伟, EvgeniiTikhonov. Hf-Ta-C-N四元化合物的结构、力学及电子性质的第一性原理研究[J]. 计算物理, 2023, 40(1): 40-46. |
| [2] | 潘靖, 沈国华. 等价阴-阳离子共掺杂调节ZnO的能带结构及其光催化活性[J]. 计算物理, 2021, 38(3): 371-378. |
| [3] | 赵一程, 郭俊宏, 胡芳仁. 应变对单层砷烯结构拉曼散射的影响[J]. 计算物理, 2020, 37(3): 365-370. |
| [4] | 熊宗刚, 杜娟, 张现周. 二维GeSe纳米片五族和七族原子掺杂的受主和施主杂质态[J]. 计算物理, 2019, 36(6): 733-741. |
| [5] | 李永, 李海生, 李冠亚, 王赵武, 李国岭, 左正伟, 李立本. 合金效应加强水分子在PtRun团簇上的吸附作用[J]. 计算物理, 2017, 34(2): 230-236. |
| [6] | 张若兴, 侯士敏, 丑强. 基于第一性原理量子输运模拟的并行计算[J]. 计算物理, 2015, 32(6): 631-638. |
| [7] | 陈金繁, 罗超, 敖冰云, 彭丽霞, 石洁. V-Ta合金力学行为的计算与实验研究[J]. 计算物理, 2014, 31(5): 609-616. |
| [8] | 李祥然, 李丹, 王春雷, 牛原, 赵红敏, 梁春军. F原子吸附TiO2:Mn(001)稀磁半导体薄膜电子结构和磁性的第一性原理计算[J]. 计算物理, 2014, 31(1): 96-102. |
| [9] | 李磊, 李丹, 刘世勇, 赵翼. Mn掺杂的ZnS(001)表面的电子态特性[J]. 计算物理, 2010, 27(2): 293-298. |
| [10] | 王义, 徐南仙, 谢元南, 林绍明, 张世泽. 铝合金及碳酚醛平面靶在高速冲击下的动态响应[J]. 计算物理, 1997, 14(6): 808-814. |
| 阅读次数 | ||||||
|
全文 |
|
|||||
|
摘要 |
|
|||||
版权所有 © 《计算物理》编辑部
地址:北京市海淀区丰豪东路2号 邮编:100094 E-mail:jswl@iapcm.ac.cn
本系统由北京玛格泰克科技发展有限公司设计开发
