Chinese Journal of Computational Physics ›› 2024, Vol. 41 ›› Issue (2): 203-213.DOI: 10.19596/j.cnki.1001-246x.8692
Previous Articles Next Articles
Chen LIU1( ), Zhongjun LIU1,*(
), Zhongjun LIU1,*( ), Minghui ZHAO1, Qingbo AO2
), Minghui ZHAO1, Qingbo AO2
Received:2023-01-07
Online:2024-03-25
Published:2024-04-03
Contact:
Zhongjun LIU
CLC Number:
Chen LIU, Zhongjun LIU, Minghui ZHAO, Qingbo AO. Grand Canonical Monte Carlo Simulation Study of Water Adsorption Behavior and Isosteric Adsorption Heat in Carbon Nanotubes[J]. Chinese Journal of Computational Physics, 2024, 41(2): 203-213.
Add to citation manager EndNote|Ris|BibTeX
URL: http://www.cjcp.org.cn/EN/10.19596/j.cnki.1001-246x.8692
| Molecule | Molecule Parameters | |||
| Carbon[ | σ/nm | (ε/kB)/k | ρs/nm-2 | |
| 0.34 | 28.0 | 38.2 | ||
| Water[ | σ/nm | (ε/kB)/k | q/e | |
| O | 0.316 6 | 78.23 | -0.847 6 | |
| H | 0.423 8 | |||
| ROM/nm | ROH/nm | ∠HOH/° | ||
| 0.0 | 0.1 | 109.47 | ||
Table 1 Potential model parameters used in the GCMC simulations
| Molecule | Molecule Parameters | |||
| Carbon[ | σ/nm | (ε/kB)/k | ρs/nm-2 | |
| 0.34 | 28.0 | 38.2 | ||
| Water[ | σ/nm | (ε/kB)/k | q/e | |
| O | 0.316 6 | 78.23 | -0.847 6 | |
| H | 0.423 8 | |||
| ROM/nm | ROH/nm | ∠HOH/° | ||
| 0.0 | 0.1 | 109.47 | ||
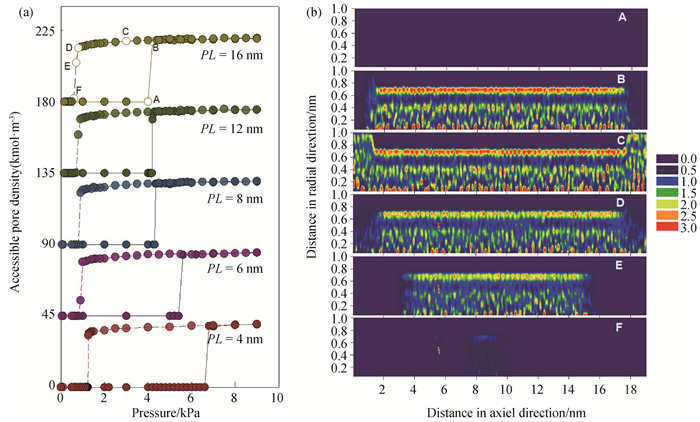
Fig.2 (a) Isotherms of water adsorption and desorption in SWCNTs with pore radius of 1.0 nm and different pore lengths at 298 K; (To ease visualization, the isotherms of PL=6, 8, 12, 16 nm are shifted up by 45, 90, 135, 180, respectively.) (b) local density distributions of water adsorbed in 1.0 nm SWCNT with pore length of 12 nm at 298 K
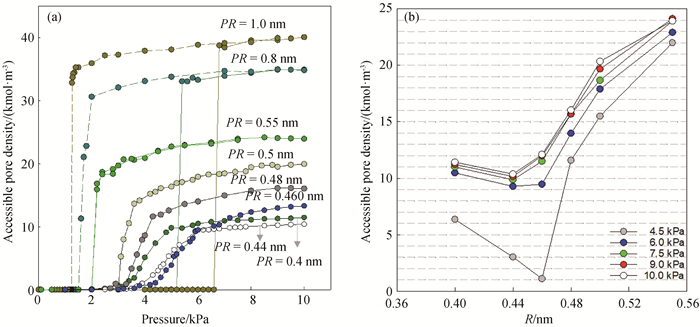
Fig.3 (a) Isotherms of water adsorption and desorption in SWCNTs with different pore radii and the pore length of 4 nm at 298 K; (For easy observation, SWCNT with PR=0.4, 0.44, 0.46, 0.48 and 0.5 nm only gives the isotherms of the adsorption branch.) (b) relations between pore densities of water adsorption and the pore radii of SWCNT at a series of pressures at 298 K
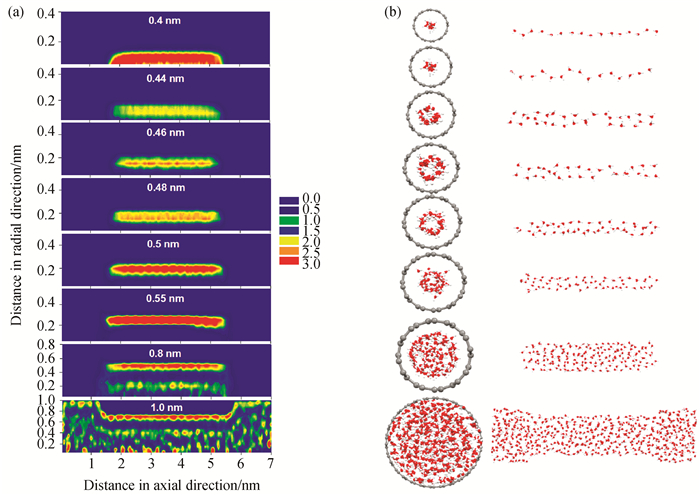
Fig.4 (a) Local density distributions of water adsorbed in SWCNTs with different pore radii and pore length of 4 nm at 298 K; (b) the snapshots of water adsorption corresponding to the adsorption conditions in (a) (The pore radius is 0.4, 0.44, 0.46, 0.48, 0.5, 0.55, 0.8, and 1.0 nm from top to bottom.)
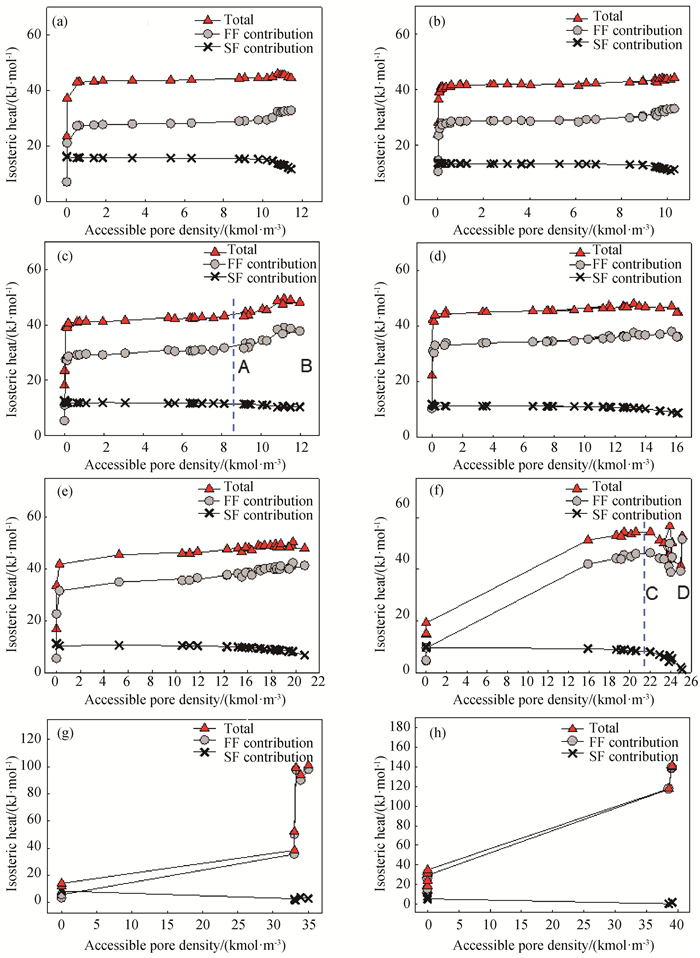
Fig.5 Isosteric heats versus accessible pore density for water adsorption at 298 K in SWCNT with different pore radius (a) PR=0.4 nm; (b) PR=0.44 nm; (c) PR=0.46 nm; (d) PR=0.48 nm; (e) PR=0.5 nm; (f) PR=0.55 nm; (g) PR=0.8 nm; (h) PR=1.0 nm
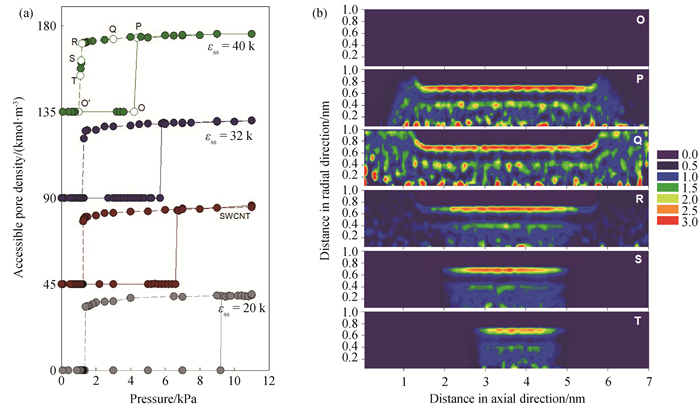
Fig.6 (a) Isotherms of water adsorbed in tubes with pore radius of 1.0 nm, pore length of 4 nm and different surface strengths of 20, 28 (SWCNT), 32, 40 K at 298 K; (To ease visualization, the isotherms of SWCNT, εss=32, 40 K are shift up by 45, 90, 135 respectively.) (b) local density distributions of water adsorbed in pores with the surface strength of 40 K
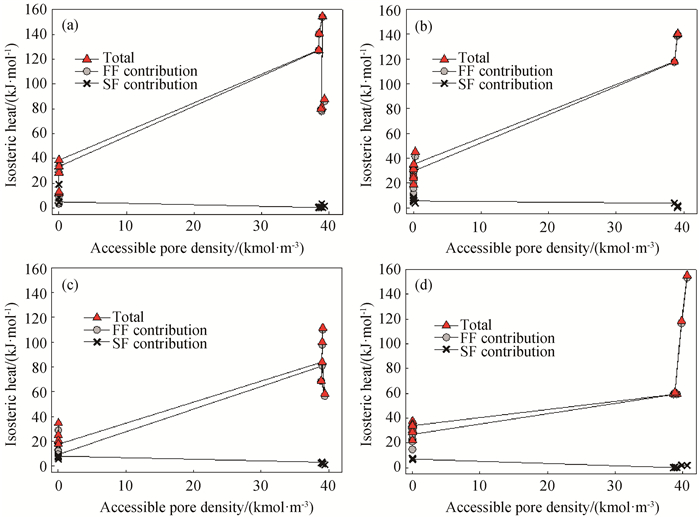
Fig.8 Isosteric heats for water adsorption in cylindrical pores with different surface strengths at 298 K (a) εss=20 K; (b) SWCNT; (c) εss=32 K; (d) εss=40 K
| 1 |
LIU L , ZENG W , TAN S J , et al. Microscopic insights into water adsorption in carbon nanopores-the role of acidic and basic functional groups and their configurations[J]. Phys Chem Phys, 2021, 23 (34): 18369- 18377.
DOI |
| 2 |
WANG C , XING Y , LEI Y , et al. Adsorption of water on carbon materials: The formation of "water bridge" and its effect on water adsorption[J]. Colloids and Surfaces A, 2021, 631, 127719.
DOI |
| 3 |
XU H , DO D D , NICHOLSON D . On the role of the quadrupole moment of carbon atom on water adsorption on graphite and in graphitic pores[J]. Chemical Engineering Journal, 2021, 409, 128236.
DOI |
| 4 |
SHI C , LIN C S , CHEN S , et al. Molecular dynamics simulation of characteristic water molecular arrangement on graphene surface and wetting transparency of graphene[J]. Acta Physica Sinica, 2019, 68 (8): 086801.
DOI |
| 5 |
HUANG Y , CHENG Q , WANG Z , et al. Competitive adsorption of benzene and water vapor on lignite-based activated carbon: Experiment and molecular simulation study[J]. Chemical Engineering Journal, 2020, 398, 125557.
DOI |
| 6 |
HORIKAWA T , YUASA R , YOSHIDA K , et al. Temperature dependence of water cluster on functionalized graphite[J]. Carbon, 2021, 183, 380- 389.
DOI |
| 7 |
OHBA T . Size-dependent water structures in carbon nanotubes[J]. Angew Chem Int Ed Engl, 2014, 53 (31): 8032- 8036.
DOI |
| 8 |
FARIMANI A B , ALURU N R . Spatial diffusion of water in carbon nanotubes: From fickian to ballistic motion[J]. J Phys Chem B, 2011, 115 (42): 12145- 12149.
DOI |
| 9 | LIU B , SHI J Q , SHEN Y , et al. A molecular dynamics simulation of methane adsorption in graphite slit-pores[J]. Chinese Journal of Computational Physics, 2013, 30 (5): 692- 699. |
| 10 | HE M C , HU X X , ZHAO J . First-principles study of carbon monoxide adsorption liability on graphite (001)[J]. Chinese Journal of Computational Physics, 2016, 33 (6): 737- 742. |
| 11 | FENG L L , XU H T , WANG D , et al. Lattice Boltzmann simulation of formaldehyde adsorption by activated carbon[J]. Chinese Journal of Computational Physics, 2021, 38 (1): 69- 78. |
| 12 |
WANG Y , DO D D , HERRERA L F , et al. On the condensation/evaporation pressures and isosteric heats for argon adsorption in pores of different cross-sections[J]. Colloids and Surfaces A, 2013, 420, 96- 102.
DOI |
| 13 |
TAN S J , PRASETYO L , DO D D , et al. On the growth of argon clusters on a weak adsorbent decorated with patches[J]. Colloid Interface Sci, 2019, 537, 431- 440.
DOI |
| 14 |
TAN S J , PRASETYO L , DO D D , et al. Interplay between wetting and filling of argon adsorption in slit pores with different surface energies transition from filling in micropores to capillary condensation in mesopores[J]. Ind Eng Chem Res, 2019, 58 (51): 23294- 23303.
DOI |
| 15 | PRASETYO L , LOI Q K , TAN S J , et al. Effects of temperature on the transition from clustering to layering for argon adsorption on substrates of different strength-parametric map of wetting, pre-wetting and non-wetting[J]. Microporous and Mesoporous Materials, 2020, 304, 30648- 30656. |
| 16 |
LOI Q K , XU H , DO D D , et al. On the description of argon adsorption on graphite for temperatures below the 2D-critical temperature[J]. Colloids and Surfaces A, 2021, 622, 126690.
DOI |
| 17 |
SONG W , YAO J , MA J , et al. Grand canonical Monte Carlo simulations of pore structure influence on methane adsorption in micro-porous carbons with applications to coal and shale systems[J]. Fuel, 2018, 215, 196- 203.
DOI |
| 18 |
LIU Y , WILCOX J . Effects of surface heterogeneity on the adsorption of CO2 in microporous carbons[J]. Environ Sci Technol, 2012, 46 (3): 1940- 1947.
DOI |
| 19 | CHIALVO A A , VLCEK L , CUMMINGS P T . Compounding effects of fluid confinement and surface strain on the wet-dry transition, thermodynamic response, and dynamics of water-graphene systems[J]. Molecular Physics, 2014, 113 (9-10): 1033- 1042. |
| 20 |
NGUYEN P T M , DO D D , NICHOLSON D . On the hysteresis loop of argon adsorption in cylindrical pores[J]. The Journal of Physical Chemistry C, 2011, 115 (11): 4706- 4720.
DOI |
| 21 |
BERENDSEN H J C , GRIGERA J R , STRAATSMA T P . The missing term in effective pair potential[J]. Phys Chem, 1987, 91 (24): 6269- 6271.
DOI |
| 22 |
MAHONEY M W , JORGENSEN W L . A five-site model for liquid water and the reproduction of the density anomaly by rigid, nonpolarizable potential functions[J]. The Journal of Chemical Physics, 2000, 112 (20): 8910- 8922.
DOI |
| 23 |
STEELE W A . The physical interaction of gases with crystalline solids: Ⅰ. Gas-solid energies and properties of isolated adsorbed atoms[J]. Surface Science, 1973, 36 (1): 317- 352.
DOI |
| 24 | PAN H , RITTER J A , BALBUENA P B . Examination of the approximations used in determining the isosteric heat of adsorption from the Clausius-Clapeyron equation[J]. Langmuir, 1998, 14 (21): 63230- 63277. |
| 25 | GARROD C . Statistical mechanics and thermodynamics[M]. New York: Oxford University Press, 1995: 622. |
| 26 |
DO D D , DO H D , NICHOLSON D . Molecular simulation of excess isotherm and excell enthalpy change in gas-phase adsorption[J]. The Journal of Physical Chemistry B, 2009, 113 (4): 1030- 1040.
DOI |
| 27 | GELB L D . The ins and outs of capillary condensation in cylindrical pores[J]. Molecular Physics, 2009, 100 (13): 2049- 2057. |
| 28 | 赵明慧, 刘忠军, 姬帅, 等. 超临界氮气在单壁碳纳米管内吸附行为的GCMC模拟研究[J]. 物理学报, 2022, 71 (22): 12- 21. |
| 29 |
DO D D , HERRERA LF , DO H D . A new method to determine pore size and its volume distribution of porous solids having known atomistic configuration[J]. Colloid Interface Sci, 2008, 328 (1): 110- 119.
DOI |
| 30 | 孙志伟, 何燕, 唐元政. 单壁碳纳米管受限空间内水的分布[J]. 物理学报, 2021, 70 (6): 151- 158. |
| 31 | EVERETT D H , POWL J C . Adsorption in slit-like and cylindrical micropores in the Henry's law region[J]. Journal of the Chemical Society, 1976, 72 (1): 619- 636. |
| [1] | Yu LI, Huiqing LIU, Yabin FENG, Xiaohu DONG, Qing WANG, Bo ZHANG. Adsorption Behavior of Heavy Oil on Montmorillonite Surface by Typical Surfactant: Molecular Dynamics Simulation [J]. Chinese Journal of Computational Physics, 2023, 40(5): 583-596. |
| [2] | Yuanqiang ZHU, Saisai JIN, Qingqing SUN. Adsorption Characteristics of N2 on Shale Kerogen [J]. Chinese Journal of Computational Physics, 2021, 38(6): 707-712. |
| [3] | Renyi CAO, Tao HUANG, Linsong CHENG, Zhanwu GAO, Zhihao JIA. Influence of Polar Substance of Crude Oil on Adsorption and Wettability in Water Flooding Reservoir: Molecular Simulation [J]. Chinese Journal of Computational Physics, 2021, 38(5): 595-602. |
| [4] | Qingqun LUO, Jiansu LI. Stability Mechanism of Adsorbed Gas in Gas-Liquid Mixed Layer [J]. Chinese Journal of Computational Physics, 2021, 38(4): 465-469. |
| [5] | FENG Lingling, XU Hongtao, WANG Di, LUO Zhuqing. Lattice Boltzmann Simulation of Formaldehyde Adsorption by Activated Carbon [J]. CHINESE JOURNAL OF COMPUTATIONAL PHYSICS, 2021, 38(1): 69-78. |
| [6] | WANG Zimo, LI Ling. Lattice Boltzmann Simulation of Fast Phase Change in Ultrashort Laser Drilling [J]. CHINESE JOURNAL OF COMPUTATIONAL PHYSICS, 2020, 37(3): 299-306. |
| [7] | CHAI Rukuan, LIU Yuetian, YANG Li, ZHANG Yixin, XIN Jing, MA Jing. Adsorption Mechanism of Two Organic Molecules with Different Polarities on Calcite (104) Surface: Density Functional Theory Study [J]. CHINESE JOURNAL OF COMPUTATIONAL PHYSICS, 2020, 37(2): 221-230. |
| [8] | CHAI Rukuan, LIU Yuetian, WANG Junqiang, XIN Jing, PI Jian, LI Changyong. Molecular Dynamics Simulation of Wettability of Calcite and Dolomite [J]. CHINESE JOURNAL OF COMPUTATIONAL PHYSICS, 2019, 36(4): 474-482. |
| [9] | WU Yan, LIU Siqi, LI Shengqiang. Electrostatic Trap and Microtrap Arrays for Cold Polar Molecules on a Chip Surface [J]. CHINESE JOURNAL OF COMPUTATIONAL PHYSICS, 2019, 36(4): 483-490. |
| [10] | LUO Zhuqing, LOU Qin, XU Hongtao, YANG Mo. LBM Simulation of Heat and Mass Double Diffusion, Fluid-Solid Conjugate Heat Transfer and Adsorption [J]. CHINESE JOURNAL OF COMPUTATIONAL PHYSICS, 2019, 36(1): 60-68. |
| [11] | ZHU Jianyu, HUANG Meng. Numerical Simulation of Fission Neutron Angular Correlation Distributions [J]. CHINESE JOURNAL OF COMPUTATIONAL PHYSICS, 2018, 35(4): 429-436. |
| [12] | YANG Longfei, ZHANG Yexin, DU Shiyu. Study of Chemical Reactivity of Doped Carbon Nanotubes by Simple Hückel Molecular Orbital Calculations with Matlab Programming [J]. CHINESE JOURNAL OF COMPUTATIONAL PHYSICS, 2017, 34(6): 685-696. |
| [13] | LI Shengqiang. A Controllable Electrostatic Well for Cold Polar Molecules in Weak Field Seeking State on a Chip [J]. CHINESE JOURNAL OF COMPUTATIONAL PHYSICS, 2017, 34(6): 731-739. |
| [14] | LI Yong, LI Haisheng, LI Guanya, WANG Zhaowu, LI Guoling, ZUO Zhengwei, LI Liben. Alloy Effects Strengthen Adsorption of H2O on PtRun Clusters [J]. CHINESE JOURNAL OF COMPUTATIONAL PHYSICS, 2017, 34(2): 230-236. |
| [15] | ZHOU Shuang, LIU Guili, JIANG Yan, SONG Yuanyuan. Adsorbing of Magnesium on Phosphorus-Doping Single-Walled Silicon Nanotubes: First-principles Study [J]. CHINESE JOURNAL OF COMPUTATIONAL PHYSICS, 2016, 33(5): 554-560. |
| Viewed | ||||||
|
Full text |
|
|||||
|
Abstract |
|
|||||
Copyright © Chinese Journal of Computational Physics
E-mail: jswl@iapcm.ac.cn
Supported by Beijing Magtech Co., Ltd.
